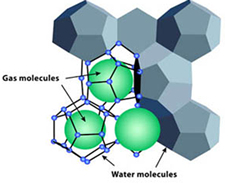
Gas Hydrates
Gas hydrates are solid crystals which resemble ice crystals. Gas molecules are trapped inside the solid water crystal. This is known as a clathrate. A clathrate is when a chemical substance consisting of a lattice of one type of molecule that traps and contains another molecule. In a gas hydrate, the trapped gas supports the crystal structure or the structure would collapse.
Oil & Gas Industry
In the oil & gas industry, gas hydrates or most commonly found where there are large pressure drops in the gas stream and as well as in pipelines. Depending on the conditions, hydrates can form at the wellhead choke valve where the Joule-Thomson effect may cause the gas temperature to fall below the temperature where hydrate formation begins. Hydrates may also form in pipelines due to the pressure and temperature drop as the gas flows down the pipeline. Hydrate formation can cause problems in gas systems as hydrate crystals can block the flow of gas.
The most common was to prevent gas hydrate formation are:
- Maintain a temperature & pressure above the hydrate formation curve for the gas. This is commonly done by heating the gas. The gas can be heated using a line heater before the gas is sent down a pipeline.
- Hydrate inhibitors can be used. The most common inhibitor is methanol. Methanol can be into the well bore or into the gas stream to prevent hydrate crystals from forming.
- Dehydrating the gas. The gas can be dehydrated using a dehydration unit. Dehydration units typically use glycol to remove the water from the gas stream. Once the water is removed the stream, hydrates can not form.
Associated Categories |
| Gas Hydrate Inhibitors |
| Line Heaters |
| Dehydrators |
Comments regarding